Application of germ-free animal models in intestinal microbiome research
History of the development of germ-free mice
The concept of germ-free animals was first proposed by Louis Pasteur in 1885, but it was not until 1946 that Reynier successfully bred the first batch of germ-free mice (Yi & Li., 2012). In the early days, germ-free mice were kept in bulky and expensive stainless steel isolators, which were later gradually replaced by lighter, easier-to-observe transparent plastic materials. Each isolator for breeding germ-free mice has an independent air flow filtration system, special transportation equipment and sterilization procedures. Therefore, although the germ-free mouse model has a history of development for some time, the establishment and maintenance of a germ-free mouse center still requires considerable manpower and technical costs because its breeding, breeding, operation and quality control processes are complicated and difficult to simplify (Lundberg et al., 2016).
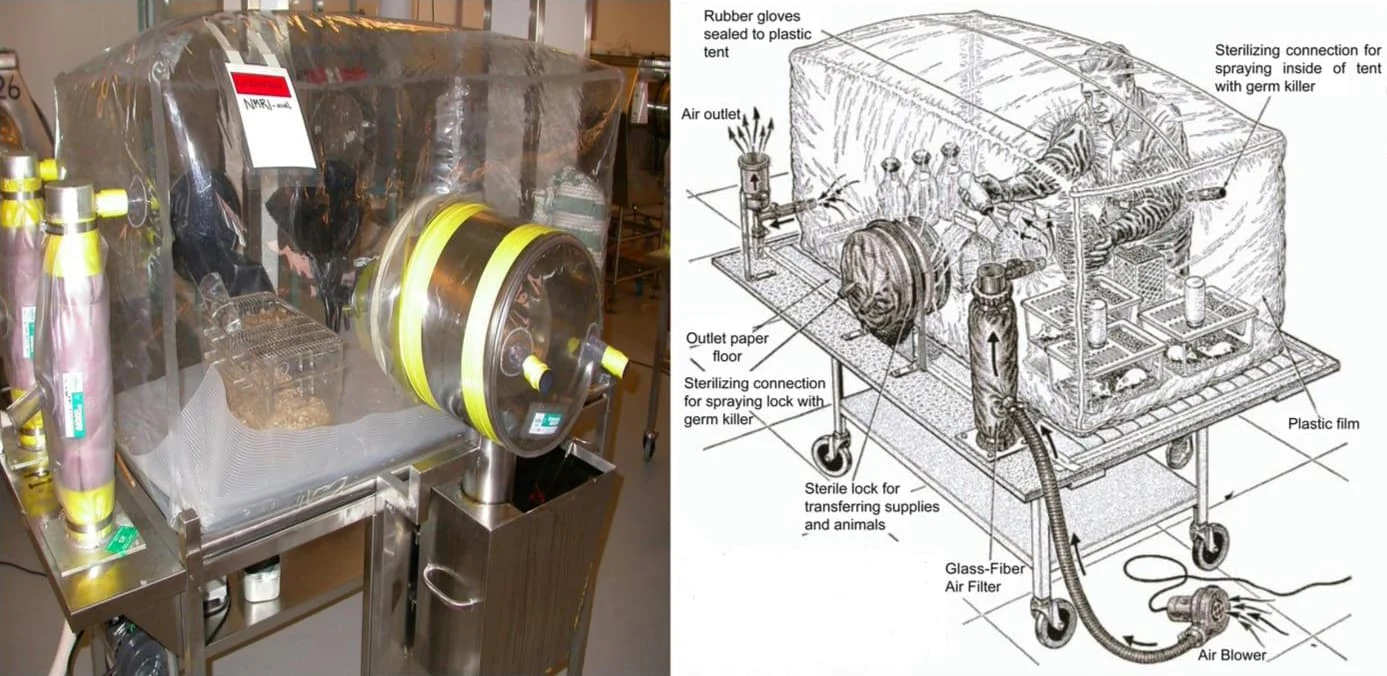
Figure 1. Sterile isolation operation box (Al-Asmakh & Zadjali., 2015)
Application of germ-free animal models in intestinal microbiome research
- Simplified Microbial Consortia
In the past, due to the high cost of gene sequencing and incomplete intestinal bacterial genome databases, most experiments on germ-free mice were conducted by sterilizing specific disease model animals (e.g. IL10-/- GFM for IBD model) and then giving them a defined bacterial inoculum to colonize their intestines, either by mono-colonization or simple consortia colonization, to explore the interaction between a single strain or simple consortia and the host and its impact on disease manifestations (Hormannsperger et al., 2015). These mice produced by giving germ-free mice known bacterial flora or strains are called Gnotobiotic Mice (gnostos "known" and bios "life", which can be translated into Chinese as known germ-free mice, germ-limited mice or all-born mice, indicating that the bacterial strains that coexist with these mice are known) (Rooks & Garrett., 2016). A well-known example is the Altered Schaedler Flora (ASF) mice, whose intestinal flora can be stably composed of only eight known strains (Clostridium species, Lactobacillus intestinalis, Lactobacillus murinus, Mucispirillum schaedleri, Eubacterium Plexicaudatum, Pseudoflavonifactor species, Clostridium species, Parabacteroides goldsteinii), yet the mice have normal intestinal immunity, pathogen resistance, and nutrient absorption capabilities. It is also a commonly used animal model for studying the interaction between intestinal microorganisms and the host (Aleksandar et al., 2013). - Complex Microbial Consortia
About ten years ago, the development of Next Generation Sequencing (NGS) technology lowered the threshold for studying microbial metagenomics in complex bacterial communities, allowing researchers to obtain sequences and species structures with richer information than traditional DGGE (Denaturing Gradient Gel Electrophoresis). It also led to a booming revival in the use of germ-free mouse models (Turnbaugh et al., 2009; Lundberg et al., 2016). Especially after Jeffery Gordon's laboratory published in 2006 that ob/ob obese mice could successfully transfer obesity traits to germ-free mice through fecal microbiota transplantation, the germ-free mouse model has gradually become a popular research tool for exploring the causal relationship between intestinal flora and diseases (Turnbaugh et al., 2006). In the following years, similar experimental methods have sprung up like mushrooms after rain, including diabetes, cardiovascular disease, brain and nerve disease, liver cancer, arthritis, autoimmune disease, allergic disease... Many disease phenotypes or disease susceptibility that were not previously thought to be related to intestinal bacteria can be transferred to another host through the host's intestinal bacteria (Hormannsperger et al., 2015), which also opened up a new field and direction for intestinal bacteria as a cause of chronic diseases caused by human environmental exposure factors. - Humanized Gnotobiotic Mice
However, due to differences in host species, the intestinal flora of mice and humans only have 15% similarities (Arrieta et al., 2016). Therefore, the results of animal experiments can only be regarded as an initial proof of concept of the importance of intestinal flora. A model suitable for the study of human intestinal flora still needs to be established. In 2009, Turnbaugh et al. successfully transplanted 85% (56/66) genus level taxa donor intestinal bacteria from healthy adult feces into the intestines of germ-free mice by FMT, establishing the first human microbiota associated mouse or humanized gnotobiotic mouse model. At the same time, they observed the changes in the structure of the human intestinal flora in the intestines of mice due to different diets (Turnbaugh et al., 2009). The establishment of this experimental model is of great encouragement for the study of human intestinal bacteria and disease and health manifestations. In addition to further confirming the causal relationship between human intestinal bacteria and disease manifestations and disease susceptibility, it can also obtain important information about how human intestinal flora regulates host metabolism and immunity, and allow scientists to explore the effects of various environmental factors that may interfere with intestinal bacteria on the composition and function of human intestinal bacteria in vivo. In the following years, many human disease and health manifestations were successfully reproduced and explored in well-controlled germ-free mouse models using human fecal microbiota transplantation, including insulin resistance and body fat gain in pregnant women during pregnancy (Koren et al., 2012), differences in fatness and thinness in twins as adults (Ridaura et al., 2013), the inducing factors of Kwashioker disease in Malawian children (Smith et al., 2013), intestinal bacterial susceptibility and asthma risk in infants and young children (Arrieta et al., 2015), functional studies on dysbiosis in inflammatory bowel disease (Nagao-Kitamoto et al., 2016), and the effects of micronutrient deficiency on human intestinal bacteria (Nagao-Kitamoto et al., 2017). - Human fecal microbiome mass culture collection model (Defined Complex Consortia)
Although the human-like intestinal bacterial mice produced by FMT can provide a lot of information on the study of the immune and metabolic functions between the human intestinal flora and the host, the fecal composition is too complex, the flora composition and metabolite components are highly variable, and may be affected by interfering factors such as non-bacterial organisms such as mold, viruses, and bacteriophages in the intestine. Therefore, there are still many difficulties to overcome in order to identify the key flora or strains that cause disease manifestations and susceptibility (Ahern et al., 2014). In order to more accurately define the bacterial flora in the human gut-mimicking mice, Goodman, Faith and others have published methods for the mass culture collection model of human fecal bacteria since 2011 (Arrayed Bacteria Culture Collection; Goodman et al., 2011; Faith et al., 2014), and conducted interventional studies on human gut-mimicking mice using defined complex flora (Defined Complex Consortia), so that the study of the flora that affects the host's immune and metabolic characteristics can obtain less error and more clear knowledge (Charbonneau et al., 2016; Hibberd et al., 2017).
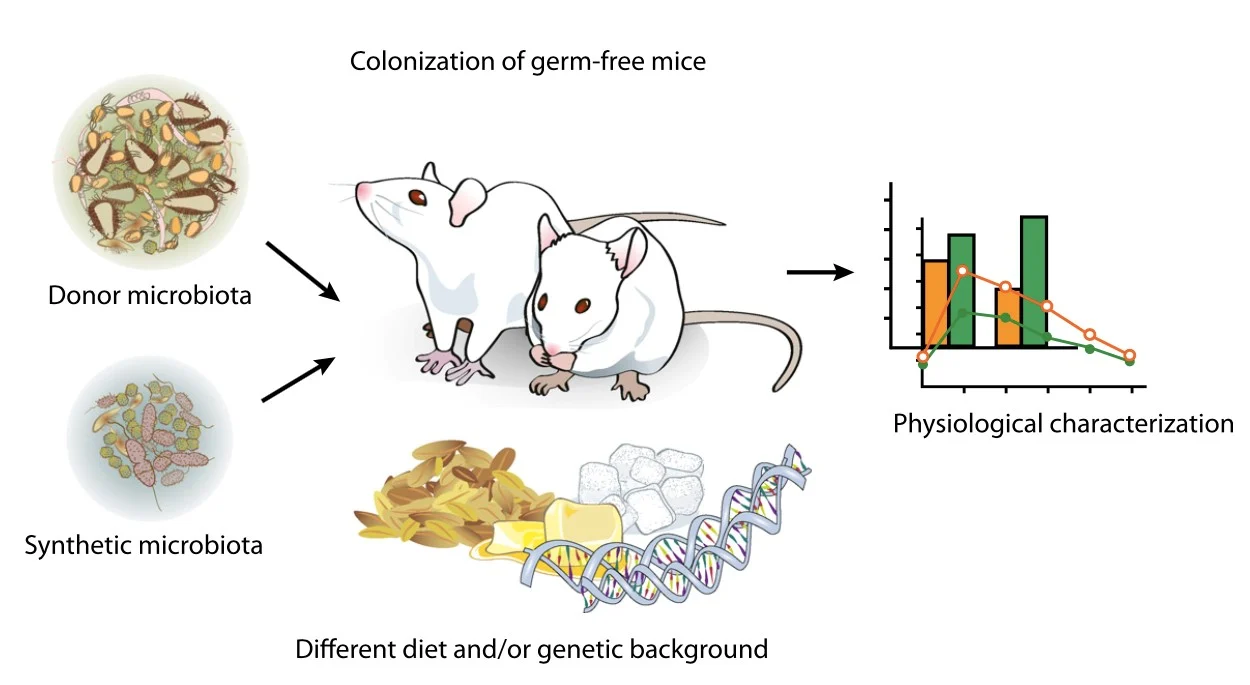
Figure 2. Basic research model of germ-free mice to explore the impact of intestinal bacteria on the host (Karlsson et al., 2013)
Challenges and limitations of germ-free mice
Even though animal models that mimic human intestinal bacteria have provided many exciting discoveries in recent years, there are still many challenges and limitations to be faced. It is generally believed that symbiotic intestinal bacteria of digestive system species have evolved together with their hosts over a long period of time, so intestinal bacteria and hosts are often specific (Arrieta et al., 2016). Chung et al. found that human flora has a poorer performance in stimulating intestinal immune maturation in germ-free mice than mouse flora, and mice exposed to human flora are more susceptible to infection by pathogens, indicating that intestinal flora and host immune maturation have their own evolutionary specificity (Chung et al., 2012). Secondly, the genes, diet, living environment and habits of mice are very different from those of humans. Their intestinal microecological environment is also different from that of humans. Some human intestinal bacteria cannot adapt to the intestinal environment of mice and therefore cannot successfully colonize. When human intestinal flora enters the intestines of mice, the composition of various microorganisms will increase or decrease due to changes in the living environment. Rawls et al. used the models of germ-free mice and germ-free zebrafish to exchange the feces of mice and zebrafish. They found that the bacterial flora successfully colonized in germ-free mice and germ-free zebrafish was still similar to the bacterial flora of the original species, indicating the importance of the ecological environment of the bacterial community to the composition of the bacterial flora (Rawls et al., 2006). Therefore, when conducting research on mouse models that mimic human intestinal bacteria, the impact of species intestinal environmental selectivity on experimental results must be considered (Arrieta et al., 2016).
Outlook
In summary, the germ-free animal model provides a well-controlled intestinal environment for intestinal bacteria research, allowing for a clearer and more in-depth discussion of the pathogenic and protective mechanisms of intestinal bacteria on the host. In particular, the application of human intestinal bacteria can still provide a lot of useful and important information about human intestinal bacteria, even with its evolutionary and ecological limitations. Although due to the progress of biotechnology, some intestinal bacteria research may be replaced by simpler in vitro intestinal bacteria models such as Gut-on-a-Chip, HuMix○R, HMI, TM gut organoid (Sonnenburg et al., 2016; von Martels et al., 2017), the germ-free mouse model has many irreplaceable features. Therefore, the author believes that this long-standing technology will still be one of the indispensable tools for intestinal bacteria research in the next 10 years.
Experts and scholars: Wu Weikai / Zhuang Xiaoli / Wu Mingxian



